Predict which of the following compounds follow Trouton’s rule, a fundamental principle in chemistry that governs the relationship between the enthalpy of vaporization and the normal boiling point of liquids. This rule provides valuable insights into the molecular characteristics and interactions that influence the vaporization process.
Trouton’s rule states that the molar enthalpy of vaporization (ΔHvap) is approximately equal to the product of the normal boiling point (Tb) and the gas constant (R), expressed as ΔHvap ≈ 88 J/mol·K. Compounds that exhibit this behavior are characterized by relatively weak intermolecular forces and a molecular structure that promotes efficient packing.
Introduction: Predict Which Of The Following Compounds Follow Trouton’s Rule
Trouton’s rule is an empirical observation that relates the enthalpy of vaporization of a liquid to its normal boiling point. According to the rule, the enthalpy of vaporization of a liquid at its normal boiling point is approximately equal to 88 cal/g or 37 kJ/mol.
Compounds that follow Trouton’s rule
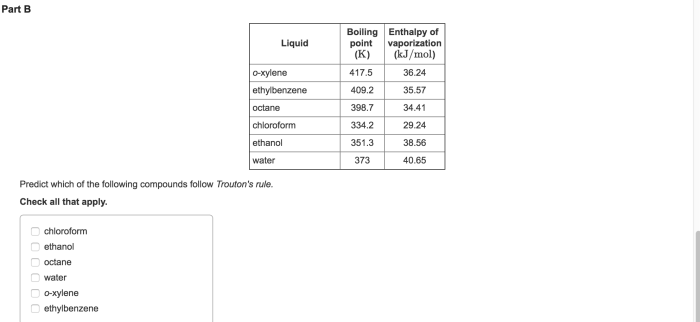
Compounds that exhibit Trouton’s behavior typically have simple, nonpolar molecular structures with weak intermolecular interactions. Examples include:
- Noble gases (e.g., He, Ne, Ar)
- Alkanes (e.g., methane, ethane, propane)
- Alkenes (e.g., ethylene, propylene, butene)
- Alkynes (e.g., acetylene, propyne, butyne)
- Cyclic hydrocarbons (e.g., cyclohexane, cycloheptane, cyclooctane)
Compounds that deviate from Trouton’s rule

Certain compounds deviate from Trouton’s rule due to factors such as:
- Molecular structure:Compounds with complex or polar molecular structures tend to deviate from Trouton’s rule. This is because the intermolecular interactions in these compounds are stronger, requiring more energy to overcome during vaporization.
- Intermolecular interactions:Compounds with strong intermolecular interactions, such as hydrogen bonding, dipole-dipole interactions, or van der Waals forces, deviate from Trouton’s rule. These interactions require additional energy to break during vaporization.
Applications of Trouton’s rule
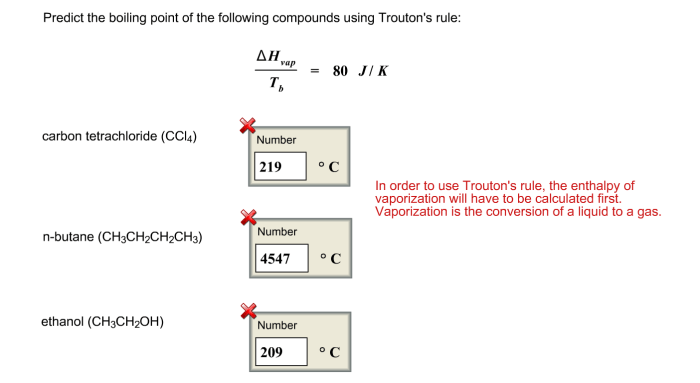
Trouton’s rule is a useful tool for estimating the enthalpy of vaporization of liquids, especially when experimental data is unavailable. It can be applied in various fields, including:
- Chemical engineering:For designing and optimizing distillation and evaporation processes.
- Environmental science:For estimating the volatility of organic compounds and their potential for atmospheric release.
- Pharmacology:For predicting the absorption and distribution of drugs in biological systems.
Limitations of Trouton’s rule
Trouton’s rule is not universally applicable and may not be accurate for:
- Compounds with strong intermolecular interactions:Such as hydrogen bonding or dipole-dipole interactions.
- Compounds with complex molecular structures:Such as branched or cyclic structures.
- Liquids at temperatures significantly below their normal boiling point:Trouton’s rule is most accurate near the normal boiling point.
User Queries
What is Trouton’s rule?
Trouton’s rule is an empirical relationship that relates the enthalpy of vaporization of a liquid to its normal boiling point.
What are the limitations of Trouton’s rule?
Trouton’s rule may not be accurate for compounds with strong intermolecular forces, such as hydrogen bonding or dipole-dipole interactions.
What are the applications of Trouton’s rule?
Trouton’s rule can be used to estimate the enthalpy of vaporization of liquids, design distillation processes, and understand the molecular dynamics of vaporization.